Effective Supplement for Slowing Macular Degeneration Progression
Researchers have recently shown that the Age-Related Eye Disease Study 2 (AREDS2) supplement formula, which replaces antioxidants zeaxanthin and lutein with beta-carotene, reduces lung cancer risks as well as more effectively combatting age-related macular degeneration progression than its original formulation.
Due to two studies showing an increased lung cancer risk among smokers when supplementing with beta-carotene, the purpose of AREDS2 was to create a supplement that was equally effective and could be taken by all regardless of smoking status. Over ten years of data collected shows not only is the AREDS2 formula safer but that it’s actually more effective in slowing the progress of age-related macular degeneration.
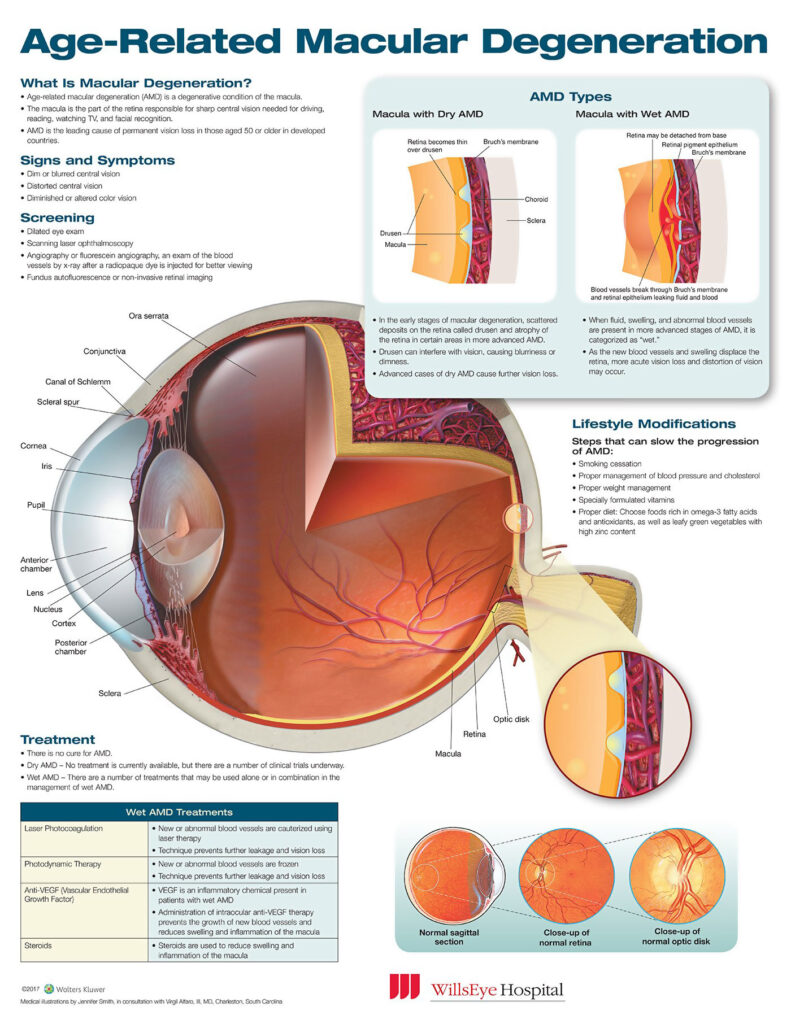
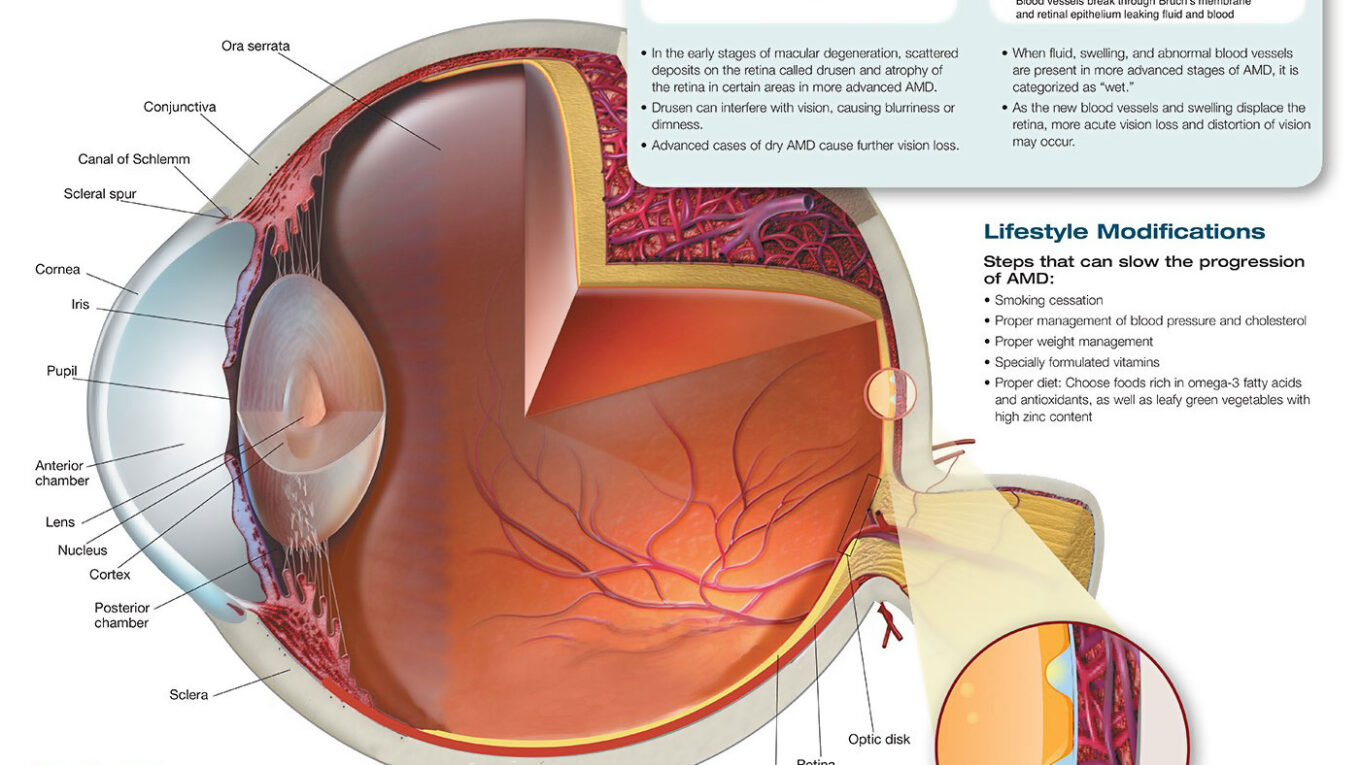
Age-related macular degeneration (AMD) is a progressive condition affecting the retina at the back of the eye. Progressive retinal cell death in the macula – providing central vision – eventually results in blindness; though treatments exist that may delay or reverse this loss. Unfortunately there is no known cure for AMD.
AREDS-1 found that taking a supplement containing 500 mg vitamin C, 15 mg beta-carotene, 400 international units of Vitamin E, 80 mg zinc and 2 mg copper could significantly slow age-related macular degeneration progression from moderate to late disease stages. Two separate studies conducted simultaneously found that smokers taking beta-carotene had significantly increased risks of lung cancer than anticipated.
The AREDS2 study compared two formulations; one containing beta-carotene to one with 2 mg zeaxanthin and 10 mg lutein instead. Both supplements are antioxidants with retinal activity; however, the formulation containing beta-carotene was only given to people who had either quit smoking or never smoked previously.
At the conclusion of the 5-year AREDS2 study, it was determined that supplementation with zeaxanthin and lutein did not increase lung cancer risks and may reduce age-related macular degeneration progression by approximately 26%. After completion, individuals received their final AREDS2 formulation consisting of zeaxanthin/lutein instead of beta-carotene.
3.883 individuals out of the initial 4,203 were followed up with five years after the AREDS2 study concluded to ascertain if age-related macular degeneration had advanced to late disease and lung cancer had been identified.
Although all individuals had switched over to taking the zeaxanthin and lutein formula after the study had ended, the follow-up study still demonstrated that beta-carotene increased lung cancer risk almost twice for individuals who ever smoked while receiving zeaxanthin and lutein had no elevated risks of lung cancer progression over 10 years when compared with those receiving beta-carotene initially. Furthermore, those originally allocated to take zeaxanthin and lutein saw an extra 20% decreased progression risk for late age-related macular degeneration when compared with individuals initially allocated beta-carotene initially.